AMCG is a cardiac diagnostic and reading solution service company.
We provide a diagnostic imaging platform using artificial intelligence and Big Data.
About AMCG All-in-one Heart Disease Diagnostic Solution
Company Introduce
-
AI-based Diagnostic Imaging Platform
- We develop a new standard system for the precise diagnosis of cardiac disease based on AI.
- We research to secure versatility of the intuitive reading using AI algorithm.
-
We have the world's best independent original technology.
- We have the world's first 96-channel magnetocardiography (MCG) system that can maximize the accuracy of diagnosis and our proprietary technology of the 2nd generation high sensitivity SQUID sensor.
- We have secured an independent original technology to re-liquefy expensive helium.
-
Early diagnosis system
- It is our mission to improve diagnostic precision..
- We will establish a highly sensitive ONE STOP early diagnosis system not even exist in the world.
Company Information
| Company Name | AMCG Co., Ltd. |
|---|---|
| CEO | Yong sung Seo |
| Founded | 2021.03.03 |
| Capital | 28billion won |
| Main Product | Medical Device Manufacturing & Medical Solution |
|---|---|
| Number of Employees | 26 |
| Location | HQ : 14F, 331, Gangnam-daero, Seocho-gu, Seoul, Republic of Korea |
Vision
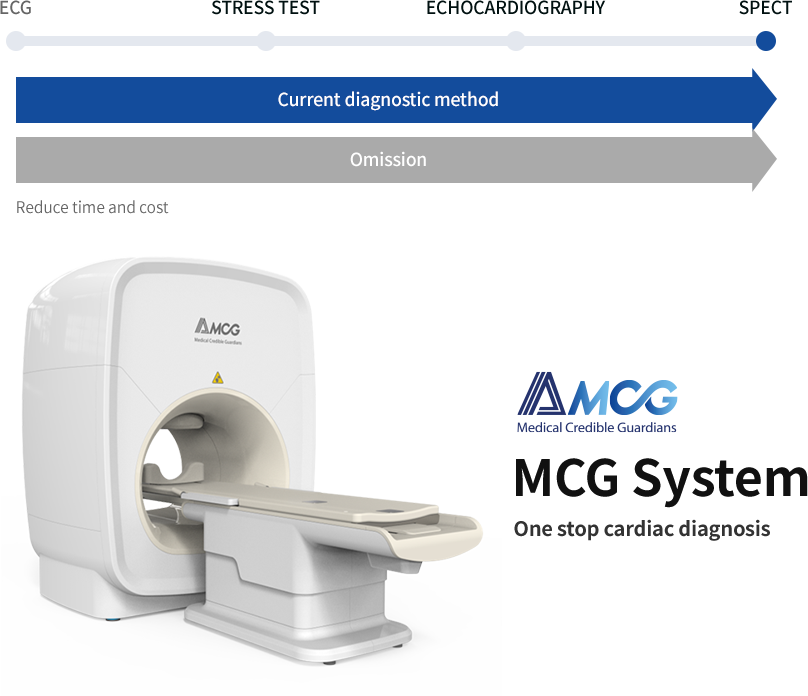
AMCG aims to develop a cardiac diagnosis system that can dramatically reduce time and cost by skipping step-by-step inspection.
-
Vision
-
Against
the LimitBeyond
the BestChallenge
the First
-
Core Value
-
For People
For Knowledge
For Future
Our Story
MCG system is a game changer in the global cardiac diagnostic testing devices market
Our History
The original technology for MCG has been successfully developed at the Korea Research Institute of Standards and Science with full support from the government for 30 years
KRISS, Korea Research Institute of Standards and Science
: Established in 1975, it is a government-appointed research institute that establishes national measurement standards and R&D advanced measurement technology.
: Established in 1975, it is a government-appointed research institute that establishes national measurement standards and R&D advanced measurement technology.
AMCG is in the progress of commercializing the MCG system
based on the original technology from KRISS.
based on the original technology from KRISS.
- Beginning Period
- Korea Research Institute of Standards and Science (KRISS) original technology development
- Promote commercialization through the supply of MCG products of the initial model and technology transfer (BMP, Germany)
- Accumulate many clinical cases using early model products and publish papers, etc.
- AMCG based on composition
- Established AMCG Co., Ltd.
- Signed a technology transfer contract between AMCG and KRISS(Korea Research Institute of Standards and Science) (2021.03)
- Established research institute (2021.04)
- Venture company certification (2021.04)
- Established GMP factory including clean room for self-production of MCG products
- Completed KFDA product license (2022.06)
- Completed production of 1st MCG product and started testing
- Promotion of Commercialization
- Clinical for accumulation of data for accurate reading
- Simultaneous clinical trials in a number of leading hospitals
- Clinical Non-Data accumulation
- Establishment of big data processing and reading system using AI
- Patient-friendly design development
- Acquired USA-FDA certification for overseas sales
- Establishment of domestic and overseas sales networks (distributors and agencies)
- Establishment of sensor factory and MCG production factory for mass production
Networks
Investors
go top






